- High diffraction efficiency when used with Agfa-Gaevaert 8E75HD emulsions.
- The low noise level.
- Color control from red through orange and yellow to green in reflection holograms exposed with red light adding one agent to the developer.
- Its suitability for reflection holograms and transmission holograms.
- The non-critical nature of the process.
- The possibility for double exposure holograms that do not suffer appreciable brightness losses.
- Technical Physical Services, Delft, The Netherlands, (R. van Renesse).
- The Holography Workshop at the Goldsmith’s College of Art (Peter Cresswell, Michael Wenyon, Bill Molteni).
- U&niversity of Ghent, Belgium (M. De Caluwe).
- Physics Laboratories, Phyllips, N.V., Eindhoven.
- University of Technology, Eindhoven (the author’s residence).
- The hardness of the gelatin. This can vary from one emulsion batch to another and is also affected by the size of the plate. Softer emulsions will collapse more readily.
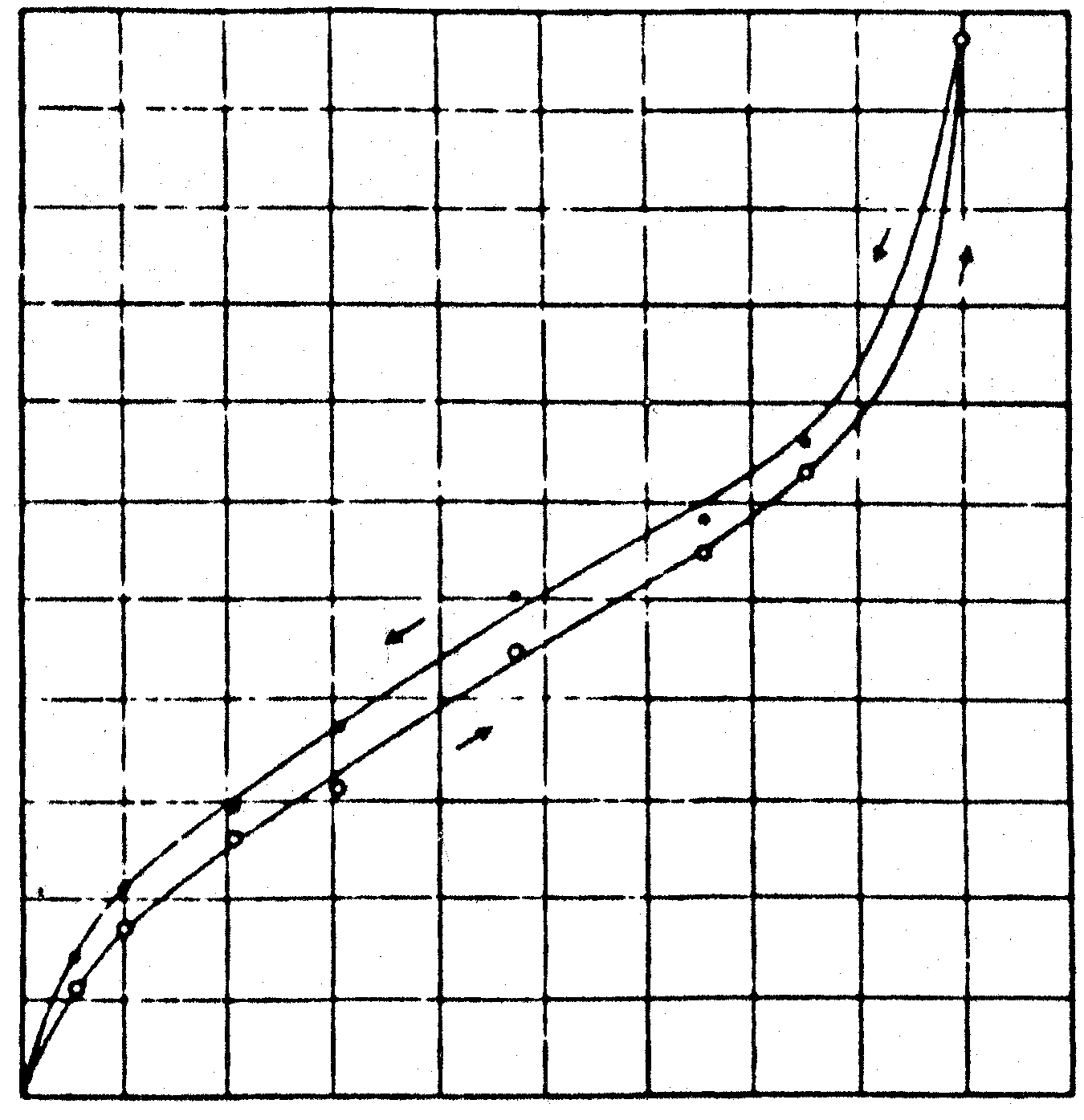
- Relative humidity. Gelatin is known to absorb water [7], the absorbed amount depending mainly upon the amount of moisture in the air (fig 1). Swelling occurs as the gelatin absorbs water. In this swollen condition the hologram is recorded. If the hologram then is viewed in an environment with a relative humidity higher or lower than that of which it was made, swelling or shrinkage can occur (on average therelative in most home and office envirionments is 50-60%). As a result the hologram can reconstruct in a color other than the desired one. (see table I).
- Developer:
-
solution A: pyrogallol 10g/l.
-
solution B: sodium carbonate anhydrous 60g/l.
- Wash for 3 minutes
- Reversal bleach
-
potassium dichromate 4g/l.
-
potassium dichromate 4g/l.
-
sulfuric acid (conc.) 4ml/l.
- Bleach till clear + 15 seconds with "maximum" of 2 minutes.
- Wash 3 minutes.
- Wash in Photo Flo 2 minutes.
- Dry: up on edge to dry on blotting paper.
- Kodak Data Release (1970).
- Beyer, H. Organic Chemistry, Verlag H. Deutsch (1963) p. 400.
- Van Renesse, Proceedings SPSE 24 1980.
- Buschman, H.T., Optik 34 245 (197).
- Joly, L. and Vanhorbeek, R.,Proceedings SPSE 24:III (1980).
- Idem 5 p. 109 and 111.
- Mees, C.E., The Theory of the Photographic Process, McMillan (1954) pp. 70-71.
- Collier, R.J., Burchhardt, C.B. and Lin, L.H.,Optical Holography, Academic Press, (1971) pp. 270-271.
- Van Renesse, R.L., Proceedings, SPSE 24 1980.
- Idem 5.
- Buschmann, H.T., Optics Commun. 6 pp. 290-294 (1972).
"Pyrochrome" Processing Yields Color-Controlled Results with Silver-Halide Materials
by
Walter Spierings,
University of Technology
Foundation Ideecentrum
Eindhoven, The Netherlands
Abstract
A well-known photographic processing system yields bright, low-noise and color-controlled results for silver halide holograms.
Introduction
This process has been published several times in the past. The Eastman Kodak Co. gave out a data release in 1970 describing it [1]. The writer learned of it from R. van Renesse and with him the following adaptation for color-controlled reflection holography was discussed.
We have named this adaptation "Pyrochrome" (from its developing and bleaching agents pyrogallol and potassium dichromate). We consider this old process to be interesting because of:
Some viewers consider the results to be comparable with some recent holograms made by N.J.Phillips. The popularity of the Pyrochrome processing is increasing rapidly through Europe. The fact that it is both of scientific and non-scientific applications, especially for artists, students, art studios, etc.
Pyrochrome processing is now in use at:
Development.
The developer is straight pyrogallol solution and is of a strang tanning nature. Pyrogallol reacts immediately with oxygen in the air in alkaline solution, [2] and it is hard to protect it from doing so. For this reason the sodium carbonate is added just before use. After mixing, the developer can be used for 10 minutes. The solution turns brown while using it due to the reaction products of pyrogallol. Because solution physical development does not occur, development time is not critical and can be between 2 and 6 minutes.
Bleaching.
The bleach is the well-known dichromate reversal bleach. It leaves the unexposed silver bromide in the gelatin to form the image. By bleaching away the silver one could think that the information disappears. To understand this it’s convenient to compare the void formed by bleaching out a silver grain to that of an air bubble formed in glass. The bubble is defined by the material surrounding it. What is needed in holography is a difference in the electrical polarization of the molecules surrounding a grain site [3]. The physical grain itself is not necessary. Bleaching is allowed for about 2 minutes. For longer times an error will occur. Some unexposed silver bromide will dissolve because of a common-ion effect with the dichromate and chromate which are undissolvable as well [4].
That is
4 AgBr(s) + Cr)42- + Cr2O72-
(Ag)2CrO4(s) + (Ag)2Cr2O7 + 4 Br-
After 2 minutes 5% of the unexposed AgBr has been dissolved; after 4 minutes 10% and after 8 minutes 20%. The unexposed silver bromide molecules help to form the image in reversal bleach, so you don’t want to loose too many of these molecules. By refreshing the bleach regularly you can get around this problem. Wash for 3 minutes.
Shrinkage Control.
Controlling gelatin shrinkage is important in holography, particularly in reflection holography because it determines the color of the image.
Holograms developed without sodium sulfite in the developer will be red if recorded with red laser light. The hologram becomes red because of a tanning effect of the conjugate pyrogallol agent. This agent tans the gelatin surrounding of a reduced silver bromide grain [5]. (Tanning is a crosslinking of gelatin molecules). A shell of relatively rigid gelatin is thus being formed around each grain.
The bleach dissolves the silver out of this shell leaving a void. The void does not shrink because its surrounding is tanned, so the gelatin keeps its original thickness, and no wavelength shift occurs.
To produce holograms which reconstruct in green, add a component that stops the tanning so the gelatin will collapse. Joly found that when adding sodium sulfite to the developer the tanning effect is lowered [6]. The conjugate pyrogallol reacts faster with the sulfite, so it can no longer tan the gelatin. The voids collapse so the emulsion will shrink and the image turns out to be green for reflection holograms recorded in red.
Colors between green and red can be obtained by adding a smaller amount of sulfite (a suggested starting point is to use 25g of sodium sulfite anhydrous for green holograms, and try smaller amounts for yellow and orange).
It is worth noting that when adding more sulfite, more unexposed silver bromide dissolves (solution physical development is indreased), and in this case a development time of two minutes is desirable.
Two other factors which effect color are:
40 36 32 28 24 20 16 12 8 4 0
Per cent
moisture44
0
10
20
30
40
50
60
70
80
90
100
per cent relative humidity
Figure 1: Relation between the moisture absorbed by dried gelatine and the relative humidity [7].
Table I
Effect of relative humidity and Sulfite Content of Developer on Color of Reconstruction in Pyrochrome Processed Reflection Holograms
Relative Humidity
40%
60%
90%
Sulfite
yes
no
yes
no
yes
no
Color
orange
red
green
red
green
green
For example, to get orange if you got green, the amount of sulfite should be lower.
Recording Material
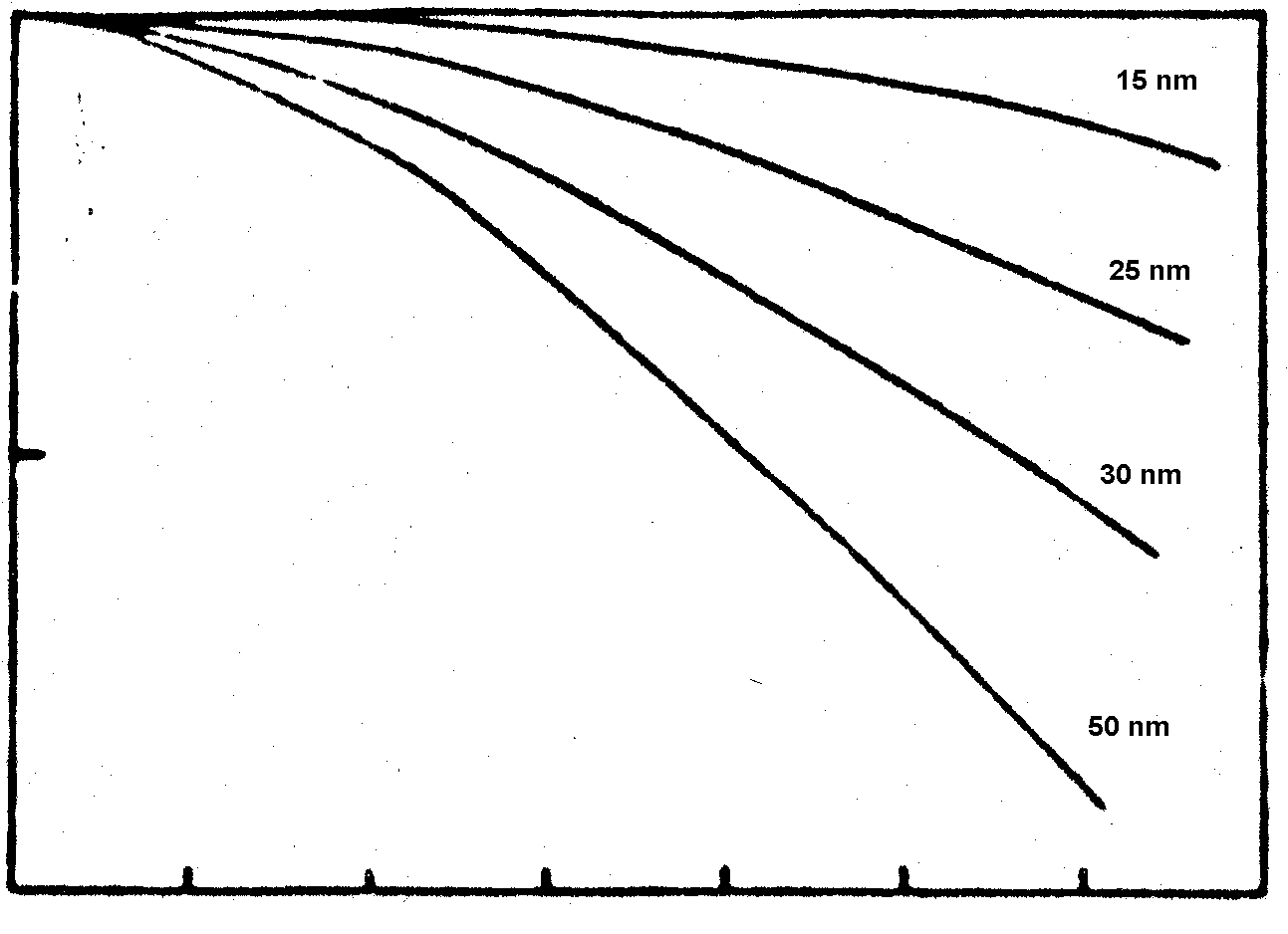
Good results were obtained with Agfa-Gaevaert 8E75HD emulsions. Figure 2 shows that especially for higher spatial frequencies the efficiency obtained with 8E75HD (35nm grain size) is four times higher than that of 8E75 (50nm grain size). The higher spatial frequencies are essential in reflection holography, where a typical resolution between 4,000 and 6,000 cycles/mm is required [7,8]. A disadvantage could be the longer exposure time required to make the recording. Depending on the batch it could be two to three times longer than 8E75.
0.5 0
Relative
diffraction
efficiency1
1000
2000
3000
4000
5000
6000
f(CYCLES/mm)
Figure 2: Calculated diffraction efficiency versus spatial frequency for different grain sizes [5].
Noise
Noise is produced by 2 major causes: scattering by the grains and reticulation of the emulsion surface [9,10].
Scattering of the grains is low because the original grains form the image. Since Pyrochrome processing employs chemical development no crystal growth effects are involved. Since grain scatter is lower for small grains, fine grain emulsions are again desirable.
Surface scattering is observed when the gelatin shrinks. Joly found that with a lower sulfite content in the developer little or no shrinkage occurs, and surface relief is small. When green holograms are sought, however, some scattering will be noticed because the gelatin has shrunk. This scatter can be minimized by index matching. A convenient way is to use plastic sprays. "Plastic 70" spray has been found to work well [11]. Available at electronic supply stores, it’s used for isolation purposes. Also, spray with black paint the surface of the reflection hologram facing away from the viewer. When this side is the emulsion side, use care, as spray paints with an acrylic base can react with the plastic spray. Slow-drying spray paints are recommended in combination with plastic spray, fast drying paints if used alone.
Exposure Time
Exposure time is not critical. Tests were carried out with exposures of 10, 20, 40 and 80 seconds on the same plate. The tests showed that there was little difference in diffraction efficiency over the range. This combined with the fact that development time can be doubled without any problem makes Pyrochrome a very easy process to use. Double exposure holograms are benefited by this combination. Very good results are obtained with reflection holograms. I refer tot the work of Peter Cresswell, head of the Goldsmith’s College of Art, London.
Process Details
References
| Back to articles |
© Dutch Holographic Laboratory